[ad_1]
Delaying surgical reconstruction of fistula structures until after patients with hidradenitis suppurativa (HS) have been on adalimumab for a minimum of 6 months transforms primary wound closure into a highly attractive option, a pilot study suggests.
“Our experience suggests that under the effects of treatment with adalimumab, wound healing disorders with primary wound closure occur less often. And primary wound closure offers advantages over secondary wound healing: shorter length of inpatient stay, lower morbidity, fewer functional problems, and better quality of life,” Gefion Girbig, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
She noted that primary wound closure following surgery for HS is controversial. For example, current German guidelines recommend complete surgical excision of HS lesions, followed by secondary wound healing; the guidelines advise against primary wound closure. But those guidelines were issued back in 2012, years before adalimumab (Humira) achieved regulatory approval as the first and to date only medication indicated for treatment of HS.
Experts agree that while adalimumab has been a difference maker for many patients with HS, surgery is still often necessary. And many surgeons prefer secondary wound healing in HS. That’s because healing by first intention has historically often resulted in complications involving wound healing disorders and infection. These complications necessitate loosening of the primary closure to permit further wound healing by second intention, with a resultant prolonged healing time, explained Girbig, of the Institute for Health Sciences Research in Dermatology and Nursing at University Medical Center Hamburg-Eppendorf (Germany).
She and her coinvestigators hypothesized that the disordered wound healing is a consequence of the underlying inflammatory disease that lies at the core of HS, and that quelling the inflammation with adalimumab for at least 6 months before performing surgery with primary closure while the anti-TNF therapy continues would reduce the incidence of wound healing disorders.
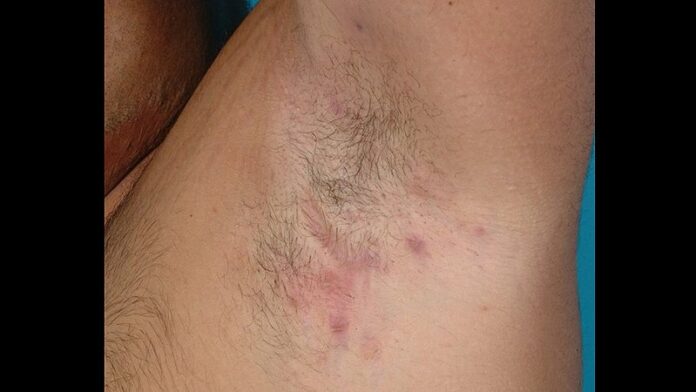
This was borne out in the group’s small observational pilot study. It included 10 patients with HS who underwent surgery only after at least 6 months on adalimumab. Six had surgery for axillary HS and four for inguinal disease. Only 2 of the 10 developed a wound healing disorder. Both had surgical reconstruction in the inguinal area. Neither case involved infection. Surgical management entailed opening part of the suture to allow simultaneous secondary wound closure.
This 20% incidence of disordered wound healing when primary closure was carried out while systemic inflammation was controlled via adalimumab is markedly lower than rates reported using primary closure without adalimumab. Girbig and her coinvestigators are now conducting a larger controlled study to confirm their findings.
Girbig reported having no financial conflicts regarding her study.
This article originally appeared on MDedge.com, part of the Medscape Professional Network.
[ad_2]
Source link