[ad_1]
Clinicians using systemic retinoids for treating ichthyosis and other disorders of cornification (DOC) in children and adolescents should aim for the lowest dose possible to balance therapeutic goals with acceptable toxicity levels, advised the authors of a new consensus statement.
In the statement, published in Pediatric Dermatology, they also addressed the effects of topical and systemic retinoid use on bone, eye, cardiovascular, and mental health, and the risks some retinoids pose to reproductive health.
Many patients with these chronic conditions, driven by multiple genetic mutations, respond to topical and/or systemic retinoids. However, to date, no specific guidance has addressed the safety, efficacy, or overall precautions for their use in the pediatric population, one of the statement authors, Moise L. Levy, MD, professor of pediatrics and medicine at the University of Texas at Austin, said in an interview.
Levy was one of the physicians on the multidisciplinary panel, The Pediatric Dermatology Research Alliance Use of Retinoids in Ichthyosis Work Group, formed to devise best practice recommendations on the use of retinoids in the management of ichthyoses and other cornification disorders in children and adolescents. The panel conducted an extensive evidence-based literature review and met in person to arrive at their conclusions. Representation from the Foundation for Ichthyosis and Related Skin Types (FIRST) was also key to this work. “Additionally, the teratogenic effects of retinoids prompted examination of gynecologic considerations and the role of the iPLEDGE program in the United States on patient access to isotretinoin,” the authors wrote.
Table of Contents
Retinoid Effects, Dosing
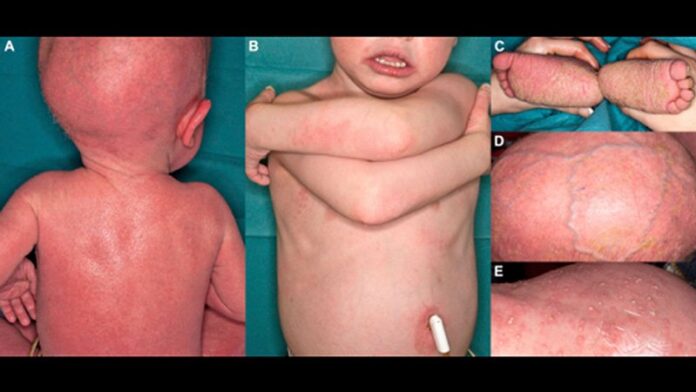
“Both topical and systemic retinoids can improve scaling in patients with select forms of ichthyosis,” and some subtypes of disease respond better to treatment than others, they noted. Oral or topical retinoids are known to improve cases of congenital ichthyosiform erythroderma (select genotypes), Sjögren-Larsson syndrome, ichthyosis follicularis–alopecia-photophobia syndromes and keratitis-ichthyosis-deafness syndrome, erythrokeratodermia variabilis, harlequin ichthyosis, ichthyosis with confetti, and other subtypes.
Comparatively, they added, there are no data on the use of retinoids, or data showing no improvement with retinoids for several ichthyosis subtypes, including congenital hemidysplasia with ichthyosiform erythroderma and limb defects, CHIME syndrome, Conradi-Hünermann-Happle syndrome, ichthyosis-hypotrichosis syndrome, ichthyosis-hypotrichosis-sclerosing cholangitis, ichthyosis prematurity syndrome, MEDNIK syndrome, peeling skin disease, Refsum syndrome, and trichothiodystrophy though the response to such cases may vary.
Retinoids may worsen conditions that lead to peeling or skin fragility, atopic diathesis, or excessive desquamation, “and should be used with caution,” the authors advised.
Pediatric and adult patients with moderate to severe disease and significant functional or psychological impairment “should be offered the opportunity to make a benefit/risk assessment of treatment” with a systemic retinoid, they added, noting that topical retinoids have a lower risk profile and may be a better choice for milder disease.
Clinicians should aim for the lowest dose possible “that will achieve and maintain the desired therapeutic effect with acceptable mucocutaneous and systemic toxicities,” the panel recommended. Lower doses work especially well in patients with epidermolytic ichthyoses and erythrokeratodermia variabilis.
“Given the cutaneous and extracutaneous toxicities of oral retinoids, lower doses were found to achieve the most acceptable risk-benefit result. Few individuals now receive more than 1 mg/kg per day of isotretinoin or 0.5 mg/kg per day of acitretin,” according to the panel.
Dosing decisions call for a group conversation between physicians, patients and caregivers, addressing skin care, comfort and appearance issues, risk of adverse effects, and tolerance of the therapy.
Retinoid Effects on Organs
The impact of retinoids on the body varies by organ system, type of therapy and dosage. Dose and duration of therapy, for example, help determine the toxic effects of retinoids on bone. “Long-term use of systemic retinoids in ichthyosis/DOC is associated with skeletal concerns,” noted the authors, adding that clinicians should still consider this therapeutic approach if there is a strong clinical case for using it in a patient.
Children on long-term systemic therapy should undergo a series of tests and evaluations for bone monitoring, including an annual growth assessment. The group also recommended a baseline skeletal radiographic survey when children are on long-term systemic retinoid therapy, repeated after 3-5 years or when symptoms are present. Clinicians should also inquire about diet and discuss with patients factors that impact susceptibility to retinoid bone toxicity, such as genetic risk, diet and physical activity.
They also recommended monitoring patients taking systemic retinoids for psychiatric symptoms.
Adolescents of childbearing potential using systemic retinoids, who are sexually active, should receive counseling about contraceptive options, and should use two forms of contraception, including one highly effective method, the statement advises.
In the United States, all patients and prescribers of isotretinoin must comply with iPLEDGE guidelines; the statement addresses the issue that iPLEDGE was not designed for long-term use of isotretinoin in patients with ichthyosis, and “imposes a significant burden” in this group.
Other practice gaps and unmet needs in this area of study were discussed, calling for a closer examination of optimal timing of therapy initiation, and the adverse effects of long-term retinoid treatment. “The work, as a whole, is a starting point for these important management issues,” said Levy.
Unrestricted educational grants from Sun Pharmaceuticals and FIRST funded this effort. Levy’s disclosed serving on the advisory board and as a consultant for Cassiopea, Regeneron, and UCB, and an investigator for Fibrocell, Galderma, Janssen, and Pfizer. The other authors disclosed serving as investigators, advisers, consultants, and/or other relationships with various pharmaceutical companies.
Pediatr Dermatol. Published online November 10, 2020. Full text
This article originally appeared on MDedge.com, part of the Medscape Professional Network.
[ad_2]
Source link