[ad_1]
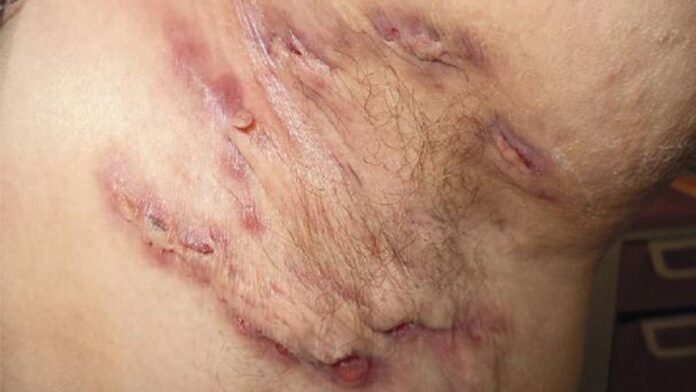
A once-daily oral selective Janus kinase 1 inhibitor demonstrated promising safety and efficacy for the treatment of moderate to severe hidradenitis suppurativa (HS) in a pair of small, randomized, phase 2 studies that established proof-of-concept for the novel agent, Afsaneh Alavi, MD, reported at the virtual annual congress of the European Academy of Dermatology and Venereology.
These favorable clinical findings were buttressed by a proteomic analysis demonstrating dose-dependent reductions in circulating inflammatory mediators, added Alavi, a dermatologist at the Mayo Clinic in Rochester, Minn.
The investigational oral small molecule, known for now as INCB54707, is 52 times more selective for JAK1 than JAK2.
Both multicenter studies entailed 8 weeks of active treatment with INCB54707 followed by a 4-week safety observation. In one study, 10 patients received 15 mg of the investigational agent once daily in open-label fashion. The other trial randomized 35 patients to the JAK1 inhibitor at 30 mg, 60 mg, or 90 mg per day or placebo. About 70% of participants in the studies had Hurley stage II HS; the rest were stage III.
Safety and tolerability were the primary outcomes in the two studies. One patient in the open-label study dropped out because of a flare of fibromyalgia. In the larger randomized trial, four patients – all in the group assigned to 90 mg/day of the JAK1 inhibitor – developed thrombocytopenia, resulting in temporary discontinuation of treatment for up to 2 weeks. In all four instances, the laboratory abnormality was reversed after temporary interruption of treatment, with no sequelae upon restarting the drug. There were no serious treatment-emergent adverse events in either study.
In the low-dose, open-label study, four of nine completers (44%) experienced a Hidradenitis Suppurativa Clinical Response (HiSCR) at week 8, defined as at least a 50% reduction in inflammatory lesion count with no increase in abscesses or draining fistulae compared to baseline. In the randomized trial, the week-8 HiSCR rate was 57% in placebo-treated controls, 56% in those on 30 mg/day or 60 mg/day of the JAK1 inhibitor, and significantly better at 88% in the group on 90 mg/day.
The rapidity of response to the JAK1 inhibitor was noteworthy. After just 1 week of treatment, an abscess and inflammatory nodule count of zero to two lesions was present in 22% of patients on INCB54707 at 60 mg/day and 29% of those on 90 mg/day, compared with none of the patients on 30 mg/day or placebo. At week 2, an abscess and nodule count of 0-2 was documented in 33% of participants on the JAK1 inhibitor at 30 mg/day, 58% at 60 mg/day, and 50% with 90 mg/day. At week 8, the rates were 57% with placebo, 44% with active treatment at 30 or 60 mg/day, and 63% in patients on 90 mg/day.
A dose-dependent significant improvement in Hidradenitis Suppurativa Quality of Life scores was documented in response to the JAK1 inhibitor.
There is an unmet need for effective therapies for HS, a chronic, extremely painful inflammatory condition with a large negative impact on quality of life. At present, the only Food and Drug Administration–approved medication for HS is the tumor necrosis factor inhibitor, adalimumab (Humira), noted Alavi. Ongoing studies are evaluating other JAK inhibitors, as well as TNF inhibitors and interleukin-17 and -23 blockers.
She reported receiving research funding from and serving as a consultant to Incyte, the studies’ sponsor, and more than a dozen other pharmaceutical companies.
This article originally appeared on MDedge.com, part of the Medscape Professional Network.
[ad_2]
Source link